RESEARCH
Current research interests include:
1) Structural basis and dynamic regulation of kinetochore assembly
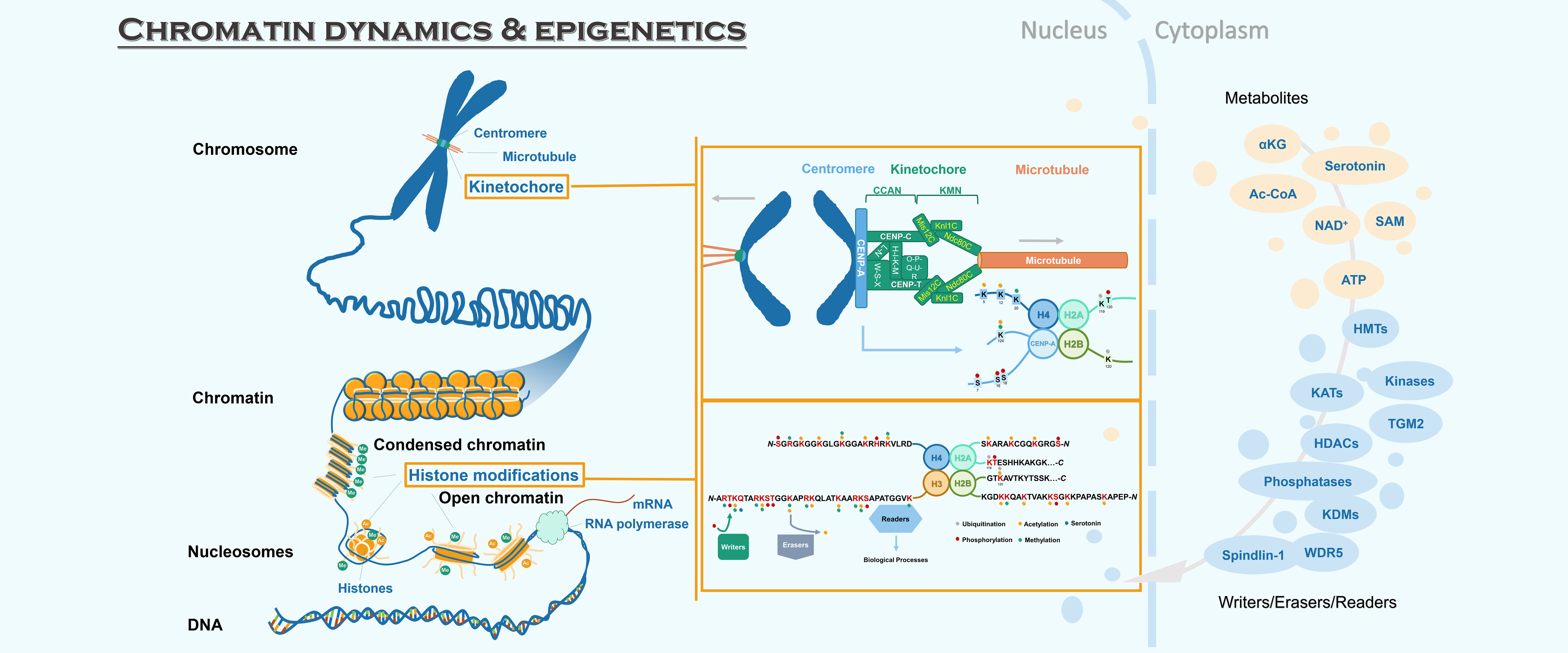
Faithful chromosome segregation during mitosis requires the correct assembly of kinetochore on centromere. CENP-A is a variant of histone H3, which specializes the centromere region on chromatin and mediates the kinetochore assembly. The centromere loading of the newly-synthesized CENP-A is mediated by the Mis18 complex. The inner kinetochore protein CENP-N and CENP-C specifically recognize CENP-A-containing chromatin and recruit other proteins to form the inner kinetochore CCAN network (CENP-LN, CENP-C, CENP-HIKM, CENP-TWSX, CENP-OPQUR) at centromere. The CCAN network links to the microtubule-binding KMN network (the KNL1 complex, Mis12 complex and Ndc80 complex) to enable accurate chromosome segregation during cell division. One direction of our lab is to explore the structural basis and dynamic regulation of kinetochore assembly, including 1) the loading of CENP-A by Mis18 complex, 2) the recognition of CENP-A-containing chromatin by CCAN network, 3) the assembly of CCAN and KMN network, and 4) the attachment of kinetochore to microtubule.
2) Molecular mechanisms underlying the physiological and pathological functions of histone modification
Posttranslational modifications on histones (hPTM) are now understood to exert major regulatory impacts on diverse biological processes. A variety of hPTMs such as methylation, acetylation, phosphorylation, and ubiquitination, has been identified to be epigenetic marks driving gene regulatory networks controlling cell fate decisions. hPTMs are dynamically regulated by ‘writer’ and ‘eraser’ enzymes, and are recognized by dozens of ‘reader’ modules, and these proteins variously coordinate to specify many distinct biological outcomes. Owing to developments in mass spectrometry technologies, new histone modifications such as crotonylation, β-hydroxybutyrylation, lactylation, and serotonylation, have been reported to activate gene transcription, and these modifications have been functionally implicated in physiological responses to metabolic stress and pathological functions in tumorigenesis. One of our research interest is to explore the molecular mechanisms underlying the regulation and recognition of hPTMs and investigate their physiological and pathological functions.