Congratulations to the publication of “SIRT2 functions as a histone delactylase and inhibits the proliferation and migration of neuroblastoma cells” in Cell Discovory.
SIRT2 functions as a histone delactylase and inhibits the proliferation and migration of neuroblastoma cells
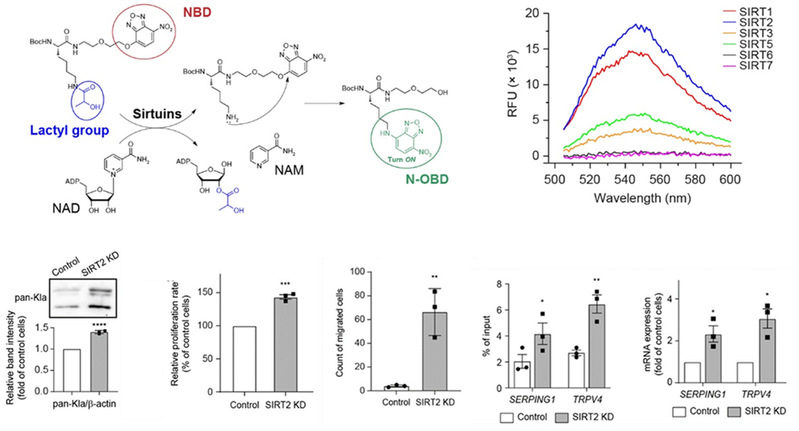
Lysine lactylation (Kla) was identified as a new type of histone acylation when breast cancer cells were under hypoxia or macrophages were exposed to bacterial challenge. Similar to other fatty acids, lactate reacts with coenzyme A to form lactyl-CoA, and Kla deposition is then catalyzed by histone acetyltransferase (HAT) p300 from a lactyl-CoA substrate, but no proteins are known to function as an “eraser” of histone lactylation modification. In this study, we identified SIRT2 as an efficient “eraser” for multiple histone lactylation sites of synthetic histone peptides, purified histones and nucleosomes, and histones in neuroblastoma cells. Moreover, we unraveled a potential regulation mechanism of aberrant histone lactylation on NB progression. Thus, agonizing SIRT2’s delactylation function may provide a unique niche for innovative antitumor therapies against neuroblastoma.
Congrats to Hanxiao Zu and Chenrui Dai for their great work. Thanks for our collaborators. Please see the full-text manuscript at https://www.nature.com/articles/s41421-022-00398-y